Legacy Healthcare announced today that the European Medicines Agency (EMA) has validated the company’s Marketing Authorisation Application (MAA) for Coacillium for the treatment of moderate and severe alopecia areata in children and adolescents.
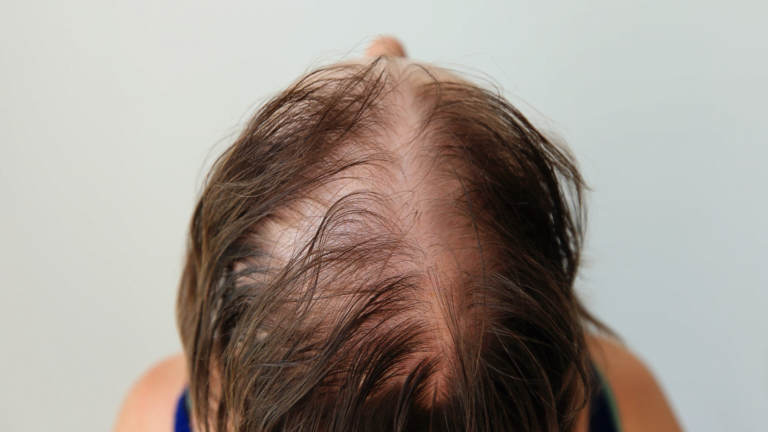
Alopecia areata is an autoimmune disorder that causes hair loss. It affects about 2% of the population, and children and adolescents are particularly vulnerable.
Coacillium is a topical treatment that is applied to the scalp. In clinical trials, Coacillium was shown to be effective in improving hair growth in children and adolescents with alopecia areata.
The validation of the MAA is an important milestone for Legacy Healthcare. It means that the EMA has accepted the company’s application for review.
The EMA will now assess the data submitted by Legacy Healthcare to determine whether Coacillium is safe and effective for the treatment of alopecia areata in children and adolescents.
If the EMA approves Coacillium, it would be the first new treatment for alopecia areata in children and adolescents in more than 20 years.
“We are very pleased that the EMA has validated our MAA for Coacillium,” said Saad Harti, CEO of Legacy Healthcare. “This is a significant milestone for our company, and we are confident that Coacillium has the potential to make a real difference in the lives of children and adolescents with alopecia areata.”
The EMA’s decision on Coacillium is expected in 2024.
Source:
- https://www.prnewswire.com/news-releases/legacy-healthcare-announces-ema-validation-of-marketing-authorisation-application-maa-for-coacillium-for-the-treatment-of-moderate-and-severe-alopecia-areata-in-children-and-adolescents-301570725.html